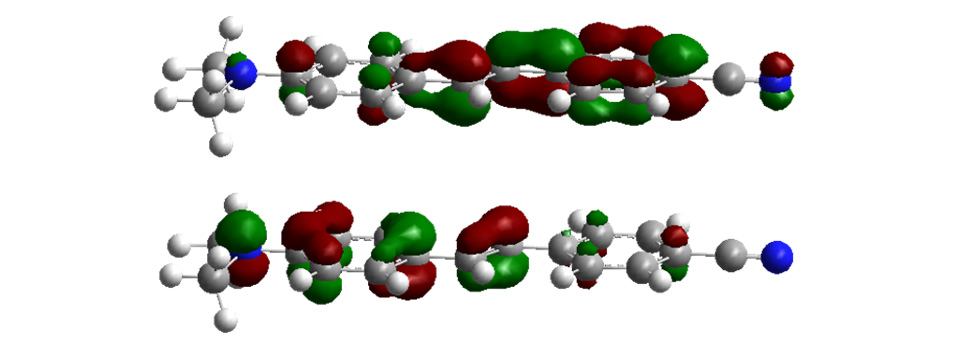
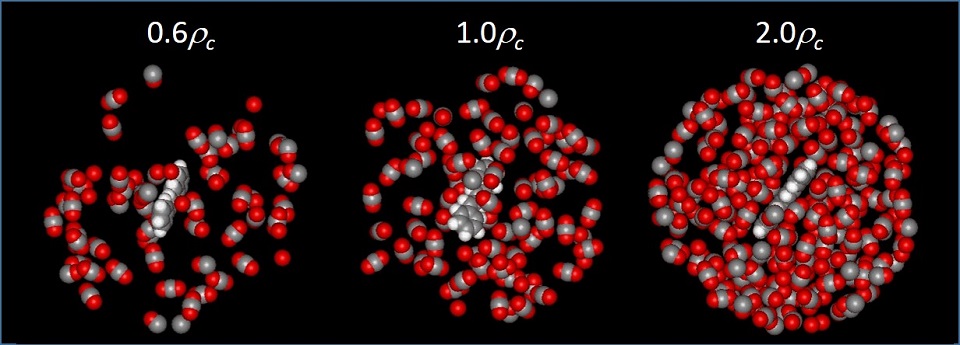
Experimental and Computational Solution Dynamics
Research in the Maroncelli group focuses on studies designed to help build a fundamental understanding of solvation and how it affects chemical reactions taking place in solution. In contrast to reactions in the gas phase, even a nominally unimolecular reaction actually involves interactions with tens to hundreds of solvent molecules. The disorder inherent to the liquid state and the rapidity of the relevant dynamics makes it difficult to describe the effect of these myriad interactions in a simple and accurate way. Our group employs state-of-the-art ultrafast spectroscopic techniques in combination with modern computational chemistry methods to help develop a molecular-level understanding of equilibrium and non-equilibrium solvation and its influence over chemistry in solution. Experimental methods mainly center around steady-state electronic spectroscopy, and ps and fs fluorescence methods. Molecular dynamics simulations and electronic structure methods provide the primary means of interpreting experimental observations. Much of our recent work has focused on elucidating the nature of solvation in unconventional solvents such as supercritical fluids, gas-expanded liquids, and ionic liquids. In these and in conventional solvents, prototypical reactions involving isomerization, electron transfer, and proton transfer are studied in order to test and develop our understanding of solvent – reaction coupling.